Does Continuous Diffusion Of Oxygen Have Potential In Chronic Diabetic Foot Ulcers?
Mark Couture, DPM, Central Texas Veteran’s Healthcare Administration, Temple, TX
Chronic wounds, typically defined as wounds that have not decreased in size by 50 percent afterfour to six weeks of treatment, are often accompanied by complications including peripheralneuropathy, diabetes, peripheral vascular disease, and poor patient adherence. 1-4
These wounds present a major clinical challenge and oxygenation is often crucial. Since impairedblood flow results in impaired oxygen supply to chronic wounds, various authors have sought tounderstand whether saturating or supersaturating wounds with oxygen can reinitiate or evenaccelerate oxygenation and healing.5-9
In a helpful overview of current pure oxygen-based therapies, Howard and colleagues concentrateon three modalities: hyperbaric oxygen therapy (HBOT), topical oxygen and continuous diffusion ofoxygen.10 All three therapies are similar in that they use pure oxygen as an aid to wound healing.Hyperbaric oxygen therapy involves systemic treatment with pure oxygen at elevated pressures.Topical oxygen therapy provides direct treatment of an area surrounding a patient’s wound, using pure oxygen at pressures slightly above atmospheric. While HBOT and topical oxygen have shownsome clinical applicability in the literature, they have two potential problems in common.
- Both therapies offer treatment for a relatively short period of time: typically 90 minutes per day, three to five days per week.
- Neither therapy allows for patient mobility during treatment. This can result in significant time and expense associated with travel and preparation for the patient.
How Continuous Diffusion of Oxygen Works
Continuous diffusion of oxygen differs from other oxygen delivery strategies in two fundamental ways. First, it provides continuous therapy, which allows for a 25-fold increase over competing intermittent strategies in the duration of oxygen delivery (continuous diffusion of oxygen is always on versus 90 minutes a day, 5 days a week for intermittent therapy).11 Second, it allows for full patient mobility, thereby reducing the risk of non-adherence and overall costs.
In effect, continuous diffusion of oxygen therapy delivers oxygen using the same basic mechanism as breathing: direct diffusion of oxygen into the wound from a wet surface. When one uses the TransCu O2® System (EO2 Concepts®), a cannula placed underneath moist wound therapy dressings delivers the oxygen directly to the wound. The moisture absorbent dressing controls exudate. The thin film dressing maintains a moist wound environment and protects the wound from external contamination.
The flow of oxygen from the device is such that it maintains a high concentration of oxygen over the moist wound but does not dry the wound out. Dressing changes occur in accordance with the physician’s directive and manufacturer’s guidelines to manage exudate. Furthermore, the TransCu O2® device is silent, lightweight (9 ounces), handheld and rechargeable. The device also incorporates continuous monitoring of oxygen flow rates and pressures to ensure efficacious delivery of oxygen. In addition to the device being able to adjust to environmental variables such as low humidity, it provides constant feedback as to the flow rate, warns of blockages and prevents over-pressurization of the wound bed.
A Closer Look At A Retrospective Analysis of 25 Cases
The Veterans Administration added TransCu O2® to its supply schedule in 2012. My colleagues and I conducted an unpublished retrospective study over a period of 16 months at a VA hospital to determine the safety and efficacy of using continuous diffusion of oxygen via the TransCu O2® in the treatment of lower extremity ulcerations that were difficult to heal (the average age of the wound at start of continuous diffusion of oxygen was almost four months). We typically begin using advanced wound therapies much earlier than four months. However, with continuous diffusion of oxygen being a new therapy in our treatment regimen, we initially tried it after the wounds were not responsive to other advanced therapies with which we were familiar.
After excluding four patients for significant non-adherence and four patients to follow-up, 25 patients were included in the study. We used continuous diffusion of oxygen both as an adjunctive therapy and as a solitary treatment in an outpatient wound care setting. The majority of wounds (22 of 25) were Wagner grade 1 ulcers on the foot, ankle, or leg with granular wound bases. Twenty-two out of 25 patients had diabetes. The patients presented on a weekly basis on average for evaluation, offloading, dressing changes and sharp debridement.
The research parameters consisted of the duration of treatment, whether the wound healed (full closure), patient adherence, wound size, percent improvement in wound size, age of wound at start of use, hemoglobin A1c level where applicable, other therapies utilized, significant comorbidities and wound locations.
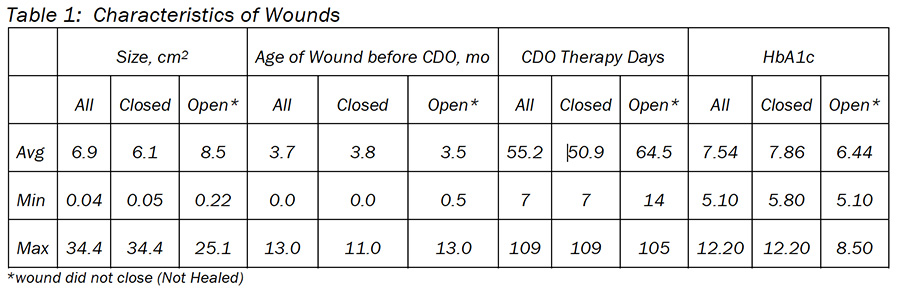
Seventeen of 25 patients experienced complete wound healing, a 68 percent healing rate. Of the 17 patients who healed (closed), all had diabetes with an average 7.9 hemoglobin A1c (Table 1). The average healing time was 7 weeks with an average wound size of 6.1 cm2. Thirteen patients had concomitant advanced tissue application: 6 with Neox (Amniox Medical), two with Dermagraft (Organogenesis), two with Apligaf (Organogenesis) and three with Grafix (Osiris Therapeutics).
These 13 patients had an average healing time of eight weeks. The four patients that healed with continuous diffusion of oxygen therapy alone had an average healing time of four weeks.
Out of the 25 patients, eight did not heal. Five of these patients had diabetes and the remaining three had ulcers due to PVD rather than diabetes. Three of these patients had no improvement during treatment, three had minimal improvement (5 to 30 percent) and two had moderate improvement (60 to 90 percent). The average wound size (8.5 cm2) was not dissimilar from those that healed. The average duration of continuous diffusion of oxygen therapy was just over nine weeks and we determined that failed therapy was a wound that had not decreased in size over the course of at least three weeks. Three patients went to another facility for HBOT as they had already failed multiple wound healing modalities. Two patients had leg amputations due to severe PVD.
Of interest is that of the three patients who were lost to follow up, two experienced significant wound closure. One was essentially healed (more than 99 percent) and did not return. The other patient’s wound had closed 89 percent before the patient was hospitalized for reasons unrelated to the wound.
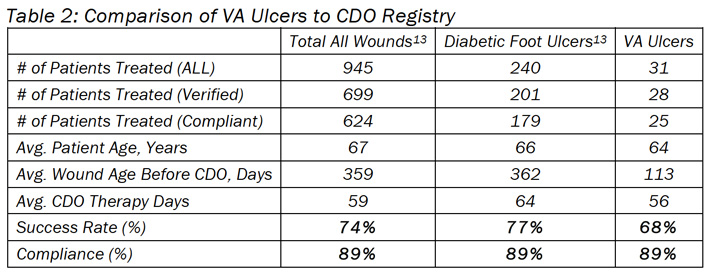
While these data support the use of continuous diffusion of oxygen as an adjunctive treatment, the fact that four patients healed with oxygen therapy alone suggests that clinicians may consider using this treatment as a monotherapy for lower extremity ulcerations. It is interesting to note that the closure results for continuous diffusion of oxygen reported here (68 percent) appear to be repeatable in that they are similar to those reported for toe ulcers (74 percent for 20 patients), as well as those reported in an online registry for diabetic foot ulcers (77 percent for 201 patients).12,13 Similar comparisons are evident for high patient adherence to the therapy, with 89 percent adherence in this study versus 95 and 89 percent adherence reported in the other studies, supporting the ease of use of the therapy.12,13
Comparison to Clinical Registry
These results compare favorably to the clinical registry that is maintained by the manufacturer, EO2 Concepts® (www.eo2.com) (Table 2). The registry contains real-world clinical outcomes that are classified as being either a Success (defined as therapy goals met or fully healed and closed) or a Failure (defined as removed for other reasons). The percentage of patients healed, CDO therapy time and compliance rates are all very similar. These outcomes are relevant in that most wounds treated had already been treated with other advanced wound therapies, such as HBO therapy, negative pressure wound therapy, skin grafting, antibiotic treatment, etc. The majority of wounds were unresponsive to other advanced wound therapies prior to treatment with CDO therapy.
In Conclusion
The emergence of continuous diffusion of oxygen therapy enables the continuous treatment of wounds through the localized diffusion of oxygen directly into the wound site. To achieve continuous treatment, the delivery device must be easily portable and reliable. Although the concept of localized diffusion of oxygen directly into wounds is not a novel technique, the manner in which the TransCu O2®
delivers the oxygen is unique to the market.
The results of this retrospective study indicate that continuous diffusion of oxygen therapy, via the TransCu O2®
>oxygen delivery system, is a viable adjunctive option in the treatment of lower extremity ulcerations. Our study findings suggest this modality may be beneficial as a solitary treatment but further studies are necessary. In my experience, continuous diffusion of oxygen improves wound healing potential, even in wounds receiving advanced tissue/skin substitute applications.
Overall, the findings presented here are in line with a significant body of experimental data suggesting that increasing oxygen supply to diabetic and chronic wounds improves wound healing. Clinicians may want to consider continuous diffusion of oxygen as part of the armamentarium for chronic lower extremity wounds.
References
1. Armstrong DG, Boulton AJM, Andros G, et al. Defining success in clinical trials of diabetic foot wounds: the Los Angeles DFCon consensus. Int Wound J. 2009; 6(3):211–213.
2. Sheehan P. Early change in wound area as a predictor of healing in diabetic foot ulcers: knowing “when to say when.” Plast Recon Surg. 2006; 117(7 Suppl):245S-247S.
3. Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Risk factors for delayed healing of neuropathic diabetic foot ulcers. Arch Dermatol 2000; 136(12):1531-1535.
4. Robson MC, Hill DP, Woodske ME, Steed DL. Wound healing trajectories as predictors of effectiveness of therapeutic agents. Arch Surg. 2000; 135(7):773-777.
5. Schreml S, Szeimies RM, Prantl L, et al. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010; 163(2):257–268.
6. Gordillo GM, Sen CK. Evidence-based recommendations for the use of topical oxygen therapy in the treatment of lower extremity wounds. Int J Lower Extr Wounds. 2009; 8(2):105-111.
7. Sen CK. Wound healing essentials – Let there be oxygen. Wound Rep Reg. 2009; 17(1):1-18.
8. Tandara AA, Mustoe TA. Oxygen in wound healing – more than nutrient. World J Surg. 2004; 28(3):294–300.
9. Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound care. Am J Surg. 2003; 186(3):259-263.
10. Howard MA, Asmis R, Evans KK, Mustoe TA. Oxygen and wound care- A review of current therapeutic modalities and future direction. Wound Rep Reg. 2013; 21(4):503-511.
11. EO2 Concepts®. Download Now.
12. Urrea-Botero G. Can Continuous Diffusion of Oxygen Heal Chronic Toe Ulcers? Podiatry Today 28(10) 2015.
13. EO2 Concepts® registry report/clinical case series review. Download Now.
Published in Podiatry Today, Vol 28, Issue 10, October 2015 (contains content not published in italics)